All medications cause side effects, but beta blockers are relatively well-tolerated in hypertensive patients.1-3 A 2017 Cochrane Review meta-analysis of beta blockers analyzed 13 randomized-controlled trials with 40,245 hypertensive patients.1 Three-quarters of the studies used atenolol, and no studies on vasodilating beta blockers were included in this analysis. The study found that those on beta blockers were no more likely than those on placebo to withdraw from treatment due to side effects (risk ratio [RR] 3.38, 95% confidence interval [CI] [0.82 – 13.95]). However, patients on propranolol or atenolol had an increased likelihood of discontinuing treatment (RR 6.35, 95% CI [3.94 – 10.22]), whereas those on oxprenolol showed no difference (RR 0.95, 95% CI [0.87 – 1.04]), when compared to the placebo group. There were no differences in the incidence of depressive symptoms (RR 1.03, 95% CI [0.65 – 1.63]), risk of fatigue (RR 4.35, 95% CI [0.17 – 108.74]), or risk of sexual dysfunction (RR 1.95, 95% CI [0.33 – 11.59]) between the beta blocker and the placebo group.
When compared to other antihypertensives, those on a beta blocker were more likely to withdraw from treatment due to side effects than those on a renin-angiotensin-system (RAS) inhibitor (RR 1.41, 95% CI [1.29 – 1.54]).1 Examples of RAS inhibitors include angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs). However, there were no significant differences found in withdrawal rates when compared to a diuretic (RR 1.69, 95% CI [0.95 – 3.00]) or a calcium-channel blocker (CCB) (RR 1.20, 95% CI [0.71 – 2.04]). Those on a beta blocker were more likely to experience fatigue than those on a diuretic (RR 2.48, 95% CI [1.73 – 3.54]), a CCB (RR 1.99, 95% CI [1.84 – 2.16]) or a RAS inhibitor (RR 1.17, 95% CI [1.06 – 1.28]). Those on a beta blocker were more likely to experience increased risk of sexual dysfunction than those on a CCB (RR 1.27, 95% CI [1.14 – 1.42]) or a RAS inhibitor (RR 1.34, 95% CI [1.10 – 1.63]). However, those on a beta blocker had a lower risk of experiencing sexual dysfunction than those on a diuretic (RR 0.50, 95% CI [0.36 – 0.70]).
A 2003 meta-analysis of 354 randomized placebo-controlled trials of hypertension treatment examined the rate of side effects of thiazide diuretics, beta blockers, ARBs, CCBs, and ACE inhibitors at standard doses.4 Similar to the Cochrane Review’s findings,1 more side effects were reported on beta blockers than on ACE inhibitors and ARBs.4 When the treatment groups were compared, 9.9% (95% CI [6.6 – 13.2]) of those on thiazides experienced one or more symptoms attributable to the drug, 8.3% (95% CI [4.8 – 11.8]) on CCBs, 7.5% (95% CI [4.0 – 10.9]) on beta blockers, 3.9% (95% CI [-0.5 – 8.3]) on ACE inhibitors, and 0.0% (95% CI [-5.4 – 5.4]) on ARBs. Although the 2017 Cochrane Review found no significant differences in adverse effects between the beta blocker and the placebo group,1 this 2003 meta-analysis found cold extremities were experienced in 2.7% (95% CI [1.4 – 4.1]) more participants in the beta blocker group than those in the placebo group, and fatigue was experienced in 1.4% (95% CI [-0.2 – 3.0]) more participants in the beta blocker group than in the placebo group.4 Side effects such as nausea, dizziness, dyspnea, and vivid dreams showed no statistically significant differences between the beta blocker and the placebo groups.
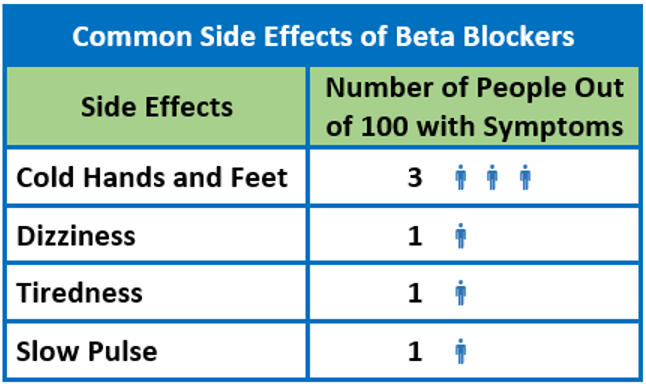
In a different study of older hypertensive women (n=315), only a few adverse events occurred at significantly different rates with the beta blocker atenolol versus an ACE inhibitor and a CCB.5 Only slow pulse occurred more often on atenolol (9%) than on ACE inhibitors (<1%) or CCB (2%) (p<0.05). Both atenolol (9%) and ACE inhibitors (7%) groups had higher rates of lower respiratory symptoms when compared to the CCB group (<1%) (p<0.05).
Side Effect Symptoms
A systematic review studying 13 randomized controlled trials of beta blockers, including bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol, in 15,383 heart failure patients found that 28 of the 33 most commonly-described side effects are not found to be significantly more common on beta blockers than on placebo.6 Only the following were significantly more common on beta blockers:
- Dizziness (19% vs. 15.3% in the placebo group, p=0.001)
- Diarrhea (21.3% vs. 17.5%, p=0.000)
- Hyperglycemia (16.1% vs. 13.4%, p=0.006)
- Claudication (2.5% vs. 1%, p=0.005)
- Bradycardia (4.9% vs. 1.6%, p=0.000)
Even so, the results show that beta blockers were genuinely responsible for dizziness, diarrhea, and hyperglycemia in less than one out of four patients who experience these side effects.6 Out of 100 patients experiencing dizziness on a beta blocker, 81 patients (95% CI [73 – 89], p<0.01) would have experienced that symptom on placebo. Similarly, 82 out of 100 patients (95% CI [70 – 95], p<0.01) experiencing diarrhea and 83 out of 100 patients (95% CI [68 – 98], p<0.01) experiencing hyperglycemia would report the symptoms on placebo. When trials of selective and non-selective beta blocker were further analyzed, only edema was significantly increased in the beta-1 selective blocker trials (p<0.01), with a borderline significant decrease in the beta-1-alpha selective blocker trials (p=0.06), showing an overall non-significant difference between placebo and beta blocker groups. Additional randomized, placebo-controlled trials on patients with heart failure showed that not many adverse events were observed significantly more often on beta blocker treatment than on placebo.7-11 Side effects expected from beta blocker, such as bradycardia, dizziness, and hypotension, were observed.
Atenolol (beta-1-selective). Manufacturers of atenolol report adverse effects studied in hypertensive patients.12,13 The following side effects are reported in more than 1%, regardless of causality: bradycardia, cold extremities, postural hypotension, leg pain, dizziness, vertigo, light-headedness, fatigue, drowsiness, depression, dreaming, diarrhea, nausea, wheezing, and dyspnea.
A randomized, double-blind study of 1,292 hypertensive men (mean age 59 ± 10 years; 48% black) compared six antihypertensive drugs and placebo for efficacy and tolerability.2 It found that the withdrawal rate due to subjective side effects for patients on atenolol (5%) was less than those of patients on captopril (7%) or placebo (6%). No side effects occurred more frequently in patients taking atenolol than placebo. A placebo-controlled, double-blind study compared atenolol to nebivolol in 336 mild-to-moderate essential hypertensive patients.14 Side effects were reported in 31% of the atenolol group, 28% of the nebivolol group, and 25% of the placebo group. When adverse effects reported at baseline were compared to that after treatment period, reports of fatigue increased and were more commonly observed in the atenolol group (n=121; baseline = 1 vs. post-intervention = 8) than in the nebivolol (n=119; 4 vs. 4) or the placebo group (n=124; 3 vs. 1).
Carvedilol (non-selective vasodilating). Manufacturers of carvedilol report adverse effects studied in more than 2,193 hypertensive patients in the U.S.15-18 The following side effects are reported in more than 1%, regardless of causality: dizziness, insomnia, diarrhea, bradycardia, postural hypotension, peripheral edema, thrombocytopenia, and hypertriglyceridemia.
In a study of 20 hypertensive men, carvedilol 50 mg and metoprolol 200 mg treatments were compared.19 Only four patients reported non-serious side effects. Two patients on metoprolol complained of reduced physical performance capacity while one on carvedilol reported feeling tired and one complained of nasal congestion. The COPERNICUS study, which included 2,289 patients with heart failure and an ejection fraction less than 25%, found that patients initiating carvedilol, compared to those on placebo, were more likely to report and have dosage decreased due to dizziness (5.8% vs. 1.9%, respectively), hypotension (3.3% vs. 1.0%), edema (1.0% vs. 0.3%), and bradycardia (2.3% vs. 0.3%) during the first eight weeks.8 However, there were no other clinically significant differences (defined as >2% difference) found between the carvedilol and the placebo group. In a separate randomized trial of 345 patients with mild-to-moderate stable chronic heart failure, only the high-dose carvedilol group reported dizziness and bradycardia more significantly.9 Groups on 12.5 mg twice a day and 25 mg twice a day reported bradycardia more commonly than the placebo group (11% vs. 1%, respectively) (p<0.05). Only the group receiving 25 mg carvedilol twice a day reported dizziness more often than the placebo group (38% vs. 23%, respectively) (p<0.05).
Metoprolol (beta-1-selective). Manufacturers of metoprolol report adverse effects studied in hypertensive patients.20,21 The following side effects are reported in more than 1%, regardless of causality: fatigue, dizziness, depression, shortness of breath, bradycardia, cold extremities, palpitations, congestive heart failure, peripheral edema, hypotension, wheezing, diarrhea, nausea, dry mouth, gastric pain, constipation, flatulence, heartburn, vomiting, and rash.
The AASK trial compared metoprolol, an ACE inhibitor, and a CCB on 1,094 African American patients with hypertensive renal disease (glomerular filtration rate 20 – 60 mL/min per 1.73m2).22 Adverse events such as angioedema and cough were experienced significantly less (p<0.05) in the metoprolol group (2.7% and 41.5%, respectively) than in the ACE inhibitor group (6.4% and 54.9%, respectively). When compared to those on CCB, less patients on metoprolol reported edema (59.8% on amlodipine vs. 51.0% on metoprolol) but more reported experiencing syncope (2.3% vs. 6.3%) (p<0.05). Efficacy and tolerability of metoprolol as a monotherapy or a combined therapy with hydrochlorothiazide were studied in 21,692 older hypertensive patients.3 Adverse effects were reported in 4.5% of the total study population, including fatigue (0.8%), dizziness (0.8%), weakness (0.4%), and nausea (0.4%). Among those who did not complete the study, 3.1% of patients withdrew from the study due to adverse experiences. The study did not report any further analysis on the occurrence of side effects, but the participating physicians noted excellent or good tolerability for 93.5% of the patients. The MERIT-HF trial studying 3,991 patients with chronic heart failure found no significant differences in the adverse events reported between the metoprolol and the placebo group, in both the diabetic (48.8% vs. 56.8%, respectively) and the non-diabetic (36.0% vs. 41.0%) groups.10 The COMET trial comparing the effect of carvedilol and metoprolol in 1,511 patients with chronic heart failure found no significant differences in the incidence of side effects and drug withdrawals between the two groups.11 However, in both the metoprolol and the carvedilol group, bradycardia (9% vs. 10%, respectively) and hypotension (11% vs. 14%, respectively) were reported commonly.
Nebivolol (beta-1-selective vasodilating). Manufacturers of nebivolol report findings on adverse effects studied in three 12-week, placebo-controlled trials with 1,597 hypertensive patients on 5 mg, 10 mg, and 20 – 40 mg of nebivolol.23 The following side effects were reported in more than 1% of patients, regardless of causality: bradycardia, diarrhea, nausea, fatigue, chest pain, peripheral edema, insomnia, headache, dizziness, chest pain, dyspnea, and rash.
A study of 913 hypertensive patients found no significant difference between the nebivolol (46.1%) and the placebo (40.7%) group in the incidence of adverse events (p=0.273).24 Only those on higher doses of nebivolol (20 or 40 mg) experienced side effects more often than those on the placebo (p<0.044 and p<0.009, respectively). Some adverse effects experienced include, headache (7.4% on placebo vs. 9.0% on 40 mg nebivolol), fatigue (2.5% vs. 4.8%), diarrhea (2.5% vs. 3.0%), dizziness (3.7% vs. 5.4%), an increased C-reactive protein level (1.2% vs. 2.4%), sinusitis (0% vs. 1.8%), upper respiratory tract infection (2.5% vs. 3.0%), peripheral edema (0% vs. 0.6%), and bronchitis (0% vs. 0.6%). Similar results were found in a different placebo-controlled study on 811 hypertensive patients.25 There were no significant differences in the incidence of adverse events among the 5 mg (39.3%) and 10 mg (39.8%) nebivolol and the placebo groups (36.0%). However, patients on 20 mg nebivolol (48.4%) experienced adverse events significantly more commonly than the placebo group (p=0.028). When the combined nebivolol group (5 mg and 20 mg) was compared to the placebo group, headache (7.5% vs, 5.3%, respectively) and fatigue (3.8% vs. 1.3%) occurred more commonly in the combined nebivolol group. Adverse effects commonly associated with beta blocker treatments such as bradycardia (0.8% vs. 0%), orthostatic hypotension (0.4% vs. 0%), dizziness (2.9% vs. 1.3%), sexual dysfunction (0.1% vs. 0%) occurred at similar rates.
References
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev 2017; 1: Cd002003.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328(13):914-921.
- LaPalio L, Schork A, Glasser S, Tifft C. Safety and efficacy of metoprolol in the treatment of hypertension in the elderly. J Am Geriatr Soc. 1992;40(4):354-358.
- Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7(31):1-94.
- Perry HM, Jr., Hall WD, Benz JR, et al. Efficacy and safety of atenolol, enalapril, and isradipine in elderly hypertensive women. Am J Med. 1994;96(1):77-86.
- Barron AJ, Zaman N, Cole GD, Wensel R, Okonko DO, Francis DP. Systematic review of genuine versus spurious side-effects of beta-blockers in heart failure using placebo control: Recommendations for patient information. Int J Cardiol. 2013;168(4):3572-3579.
- Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215-225.
- Krum H, Roecker EB, Mohacsi P, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: Results from the copernicus study. JAMA. 2003;289(6):712-718.
- Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94(11):2807-2816.
- Deedwania PC, Giles TD, Klibaner M, et al. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT-HF. Am Heart J. 2005;149(1):159-167.
- Poole-Wilson PA, Swedberg K, Cleland JGF, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7-13.
- Tenormin Injection [package insert]. Conovanas, PR: AstraZeneca Pharmaceuticals LP; 2016.
- Tenormin [package insert]. Conovanas, PR: AstraZeneca Pharmaceuticals LP; 2011.
- Van Nueten L, Taylor FR, Robertson JI. Nebivolol vs atenolol and placebo in essential hypertension: a double-blind randomised trial. J Hum Hypertens. 1998;12(2):135-140.
- Coreg CR [package insert]. Ciales, PR: GK Pharmaceuticals Contract Manufacturing Operations; 2008.
- Coreg [package insert]. Ciales, PR: GK Pharmaceuticals Contract Manufacturing Operations; 2008.
- Auro-Carvedilol [package insert]. Ontario, CA: Aurobindo Pharma Inc.; 2013.
- APO-Carvedilol [package insert]. Toronto, Canada: Apotex Pharmaceutical Holdings Inc.; 2015.
- Franz IW, Agrawal B, Wiewel D, Ketelhut R. Comparison of the antihypertensive effects of carvedilol and metoprolol on resting and exercise blood pressure. Clin Investig. 1992;70 Suppl 1:S53-57.
- Metoprolol succinate extended-release tablets [package insert]. Sodertalje, Sweden: AstraZeneca AB; 2006.
- Lopressor [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2008.
- Wright JT, Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421-2431.
- Bystolic [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2011.
- Weiss RJ, Weber MA, Carr AA, Sullivan WA. A randomized, double-blind, placebo-controlled parallel-group study to assess the efficacy and safety of nebivolol, a novel beta-blocker, in patients with mild to moderate hypertension. J Clin Hypertens. 2007;9(9):667-676.
- Greathouse M. Nebivolol efficacy and safety in patients with stage I-II hypertension. Clin Cardiol. 2010;33(4):E20-27.